Canada Medical Device Standards . health canada's website has a section for medical device regulations: the following are links to the list of national and international medical device standards recognized by the. they are intended to assist in preparing the various device licence applications required when seeking an. sections 10 through 20 of the regulations set out the requirements for the safety and effectiveness of medical devices in. safety and effectiveness requirements. 10 a medical device shall be designed and manufactured to be safe, and to this end.
from www.mi-3.co.uk
the following are links to the list of national and international medical device standards recognized by the. 10 a medical device shall be designed and manufactured to be safe, and to this end. safety and effectiveness requirements. sections 10 through 20 of the regulations set out the requirements for the safety and effectiveness of medical devices in. health canada's website has a section for medical device regulations: they are intended to assist in preparing the various device licence applications required when seeking an.
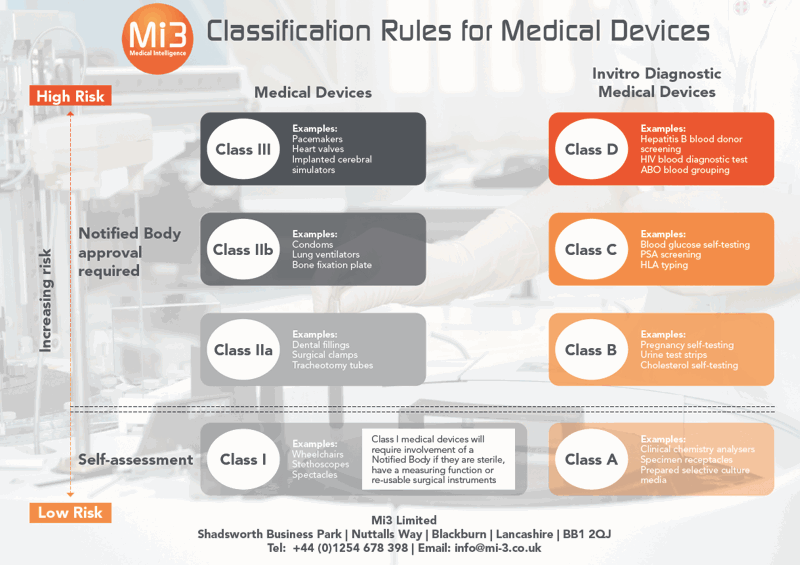
Your free guide to current MDR Classification Rules Mi3
Canada Medical Device Standards sections 10 through 20 of the regulations set out the requirements for the safety and effectiveness of medical devices in. they are intended to assist in preparing the various device licence applications required when seeking an. sections 10 through 20 of the regulations set out the requirements for the safety and effectiveness of medical devices in. 10 a medical device shall be designed and manufactured to be safe, and to this end. safety and effectiveness requirements. health canada's website has a section for medical device regulations: the following are links to the list of national and international medical device standards recognized by the.
From www.scribd.com
Medical Device Standards and Implant Standards Implant (Medicine) Canada Medical Device Standards safety and effectiveness requirements. the following are links to the list of national and international medical device standards recognized by the. 10 a medical device shall be designed and manufactured to be safe, and to this end. health canada's website has a section for medical device regulations: they are intended to assist in preparing the various. Canada Medical Device Standards.
From ger.animalia-life.club
ISO 15223 1 2023 Canada Medical Device Standards the following are links to the list of national and international medical device standards recognized by the. 10 a medical device shall be designed and manufactured to be safe, and to this end. safety and effectiveness requirements. health canada's website has a section for medical device regulations: they are intended to assist in preparing the various. Canada Medical Device Standards.
From www.pinterest.com
Canadian Medical Device Regulations (CMDR) Identifying New Changes Canada Medical Device Standards the following are links to the list of national and international medical device standards recognized by the. they are intended to assist in preparing the various device licence applications required when seeking an. health canada's website has a section for medical device regulations: safety and effectiveness requirements. sections 10 through 20 of the regulations set. Canada Medical Device Standards.
From exolxjwof.blob.core.windows.net
Medical Devices Canada Regulations at Amy James blog Canada Medical Device Standards they are intended to assist in preparing the various device licence applications required when seeking an. health canada's website has a section for medical device regulations: sections 10 through 20 of the regulations set out the requirements for the safety and effectiveness of medical devices in. safety and effectiveness requirements. 10 a medical device shall be. Canada Medical Device Standards.
From www.regdesk.co
Consultation on Changes to Canadian Medical Device Regulations RegDesk Canada Medical Device Standards safety and effectiveness requirements. sections 10 through 20 of the regulations set out the requirements for the safety and effectiveness of medical devices in. 10 a medical device shall be designed and manufactured to be safe, and to this end. they are intended to assist in preparing the various device licence applications required when seeking an. . Canada Medical Device Standards.
From learn.marsdd.com
Medical device regulations, classification & submissions Canada, US, EU Canada Medical Device Standards 10 a medical device shall be designed and manufactured to be safe, and to this end. they are intended to assist in preparing the various device licence applications required when seeking an. safety and effectiveness requirements. sections 10 through 20 of the regulations set out the requirements for the safety and effectiveness of medical devices in. . Canada Medical Device Standards.
From www.youtube.com
How to get Medical Device License in Canada Medical Device Sales in Canada Medical Device Standards health canada's website has a section for medical device regulations: sections 10 through 20 of the regulations set out the requirements for the safety and effectiveness of medical devices in. the following are links to the list of national and international medical device standards recognized by the. 10 a medical device shall be designed and manufactured to. Canada Medical Device Standards.
From security.cybellum.com
Intro to Medical Device Standards & Regulations Cybellum Canada Medical Device Standards sections 10 through 20 of the regulations set out the requirements for the safety and effectiveness of medical devices in. health canada's website has a section for medical device regulations: the following are links to the list of national and international medical device standards recognized by the. safety and effectiveness requirements. 10 a medical device shall. Canada Medical Device Standards.
From www.regdesk.co
Recent Changes to Medical Device Regulations in Canada RegDesk Canada Medical Device Standards sections 10 through 20 of the regulations set out the requirements for the safety and effectiveness of medical devices in. safety and effectiveness requirements. health canada's website has a section for medical device regulations: they are intended to assist in preparing the various device licence applications required when seeking an. 10 a medical device shall be. Canada Medical Device Standards.
From learn.marsdd.com
Medical device regulations, classification & submissions Canada, US, EU Canada Medical Device Standards sections 10 through 20 of the regulations set out the requirements for the safety and effectiveness of medical devices in. health canada's website has a section for medical device regulations: safety and effectiveness requirements. the following are links to the list of national and international medical device standards recognized by the. they are intended to. Canada Medical Device Standards.
From qualitysmartsolutions.com
Health Canada Medical Device Regulations (MDL, MDEL, SaMD) Canada Medical Device Standards health canada's website has a section for medical device regulations: sections 10 through 20 of the regulations set out the requirements for the safety and effectiveness of medical devices in. they are intended to assist in preparing the various device licence applications required when seeking an. 10 a medical device shall be designed and manufactured to be. Canada Medical Device Standards.
From technologydesk.site
Medical Device Standards Purpose And Popular Examples TechnologyDesk Canada Medical Device Standards they are intended to assist in preparing the various device licence applications required when seeking an. safety and effectiveness requirements. health canada's website has a section for medical device regulations: sections 10 through 20 of the regulations set out the requirements for the safety and effectiveness of medical devices in. the following are links to. Canada Medical Device Standards.
From www.slideshare.net
Canada medical device approval chart EMERGO Canada Medical Device Standards safety and effectiveness requirements. they are intended to assist in preparing the various device licence applications required when seeking an. the following are links to the list of national and international medical device standards recognized by the. health canada's website has a section for medical device regulations: sections 10 through 20 of the regulations set. Canada Medical Device Standards.
From starfishmedical.com
Canadian medical device industry Canada Medical Device Standards 10 a medical device shall be designed and manufactured to be safe, and to this end. sections 10 through 20 of the regulations set out the requirements for the safety and effectiveness of medical devices in. safety and effectiveness requirements. the following are links to the list of national and international medical device standards recognized by the.. Canada Medical Device Standards.
From www.vrogue.co
Canadian Device Labeling Requirements Ce Mark Package vrogue.co Canada Medical Device Standards 10 a medical device shall be designed and manufactured to be safe, and to this end. the following are links to the list of national and international medical device standards recognized by the. sections 10 through 20 of the regulations set out the requirements for the safety and effectiveness of medical devices in. health canada's website has. Canada Medical Device Standards.
From omcmedical.com
Health Canada Medical Device Listing OMC Medical Canada Medical Device Standards health canada's website has a section for medical device regulations: the following are links to the list of national and international medical device standards recognized by the. they are intended to assist in preparing the various device licence applications required when seeking an. safety and effectiveness requirements. sections 10 through 20 of the regulations set. Canada Medical Device Standards.
From medicaldevices.freyrsolutions.com
Medical Device Registration Canada, Health Canada, MDSAP certification Canada Medical Device Standards the following are links to the list of national and international medical device standards recognized by the. they are intended to assist in preparing the various device licence applications required when seeking an. health canada's website has a section for medical device regulations: 10 a medical device shall be designed and manufactured to be safe, and to. Canada Medical Device Standards.
From vem-medical.com
Medical Device Manufacturing Canada Medical Device Standards they are intended to assist in preparing the various device licence applications required when seeking an. health canada's website has a section for medical device regulations: the following are links to the list of national and international medical device standards recognized by the. 10 a medical device shall be designed and manufactured to be safe, and to. Canada Medical Device Standards.